14 Major Diseases Of The Nervous System
14.1 Neurodegenerative Diseases
Neurodegeneration is the progressive loss of structure or function of neurons, including death of neurons. Many neurodegenerative diseases – including amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, fatal familial insomnia, and Huntington’s disease – occur as a result of neurodegenerative processes. Such diseases are incurable, resulting in progressive degeneration and/or death of neurons. As research progresses, many similarities appear that relate these diseases to one another on a sub-cellular level. Discovering these similarities offers hope for therapeutic advances that could ameliorate many diseases simultaneously. There are many parallels between different neurodegenerative disorders including atypical protein assemblies as well as induced cell death. Neurodegeneration can be found in many different levels of neuronal circuitry ranging from molecular to systemic.
The greatest risk factor for neurodegenerative diseases is aging. Mitochondrial DNA mutations as well as oxidative stress both contribute to aging. Many of these diseases are late-onset, meaning there is some factor that changes as a person ages for each disease. One constant factor is that in each disease, neurons gradually lose function as the disease progresses with age. It has been proposed that DNA damage accumulation provides the underlying causative link between aging and neurodegenerative disease. About 20-40% of healthy people between 60 and 78 years old experience discernable decrements in cognitive performance in several domains including working, spatial, and episodic memory, and processing speed.
Many neurodegenerative diseases are caused by genetic mutations, most of which are located in completely unrelated genes. In many of the different diseases, the mutated gene has a common feature: a repeat of the CAG nucleotide triplet. CAG codes for the amino acid glutamine. A repeat of CAG results in a polyglutamine (polyQ) tract. Diseases associated with such mutations are known as trinucleotide repeat disorders.
Polyglutamine repeats typically cause dominant pathogenesis. Extra glutamine residues can acquire toxic properties through a variety of ways, including irregular protein folding and degradation pathways, altered subcellular localization, and abnormal interactions with other cellular proteins. PolyQ studies often use a variety of animal models because there is such a clearly defined trigger – repeat expansion. Extensive research has been done using the models of nematode (C. elegans), and fruit fly (Drosophila), mice, and non-human primates.
Nine inherited neurodegenerative diseases are caused by the expansion of the CAG trinucleotide and polyQ tract, including Huntington’s disease and the spinocerebellar ataxias.
Several neurodegenerative diseases are classified as proteopathies as they are associated with the aggregation of misfolded proteins.
- alpha-synuclein: can aggregate to form insoluble fibrils in pathological conditions characterized by Lewy bodies, such as Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Alpha-synuclein is the primary structural component of Lewy body fibrils. In addition, an alpha-synuclein fragment, known as the non-Abeta component (NAC), is found in amyloid plaques in Alzheimer’s disease.
- tau: hyperphosphorylated tau protein is the main component of neurofibrillary tangles in Alzheimer’s disease.
- beta amyloid: the major component of senile plaques in Alzheimer’s disease.
- prion: main component of prion diseases and transmissible spongiform encephalopathies.
14.1.1 Alzheimer’s disease
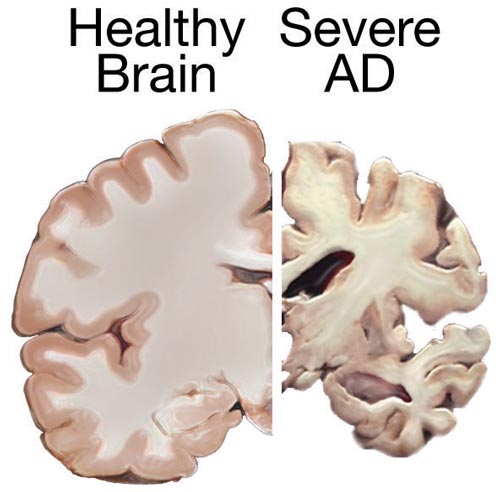
Alzheimer’s disease (AD) is a chronic neurodegenerative disease that results in the loss of neurons and synapses in the cerebral cortex and certain subcortical structures, resulting in gross atrophy of the temporal lobe, parietal lobe, and parts of the frontal cortex and cingulate gyrus.
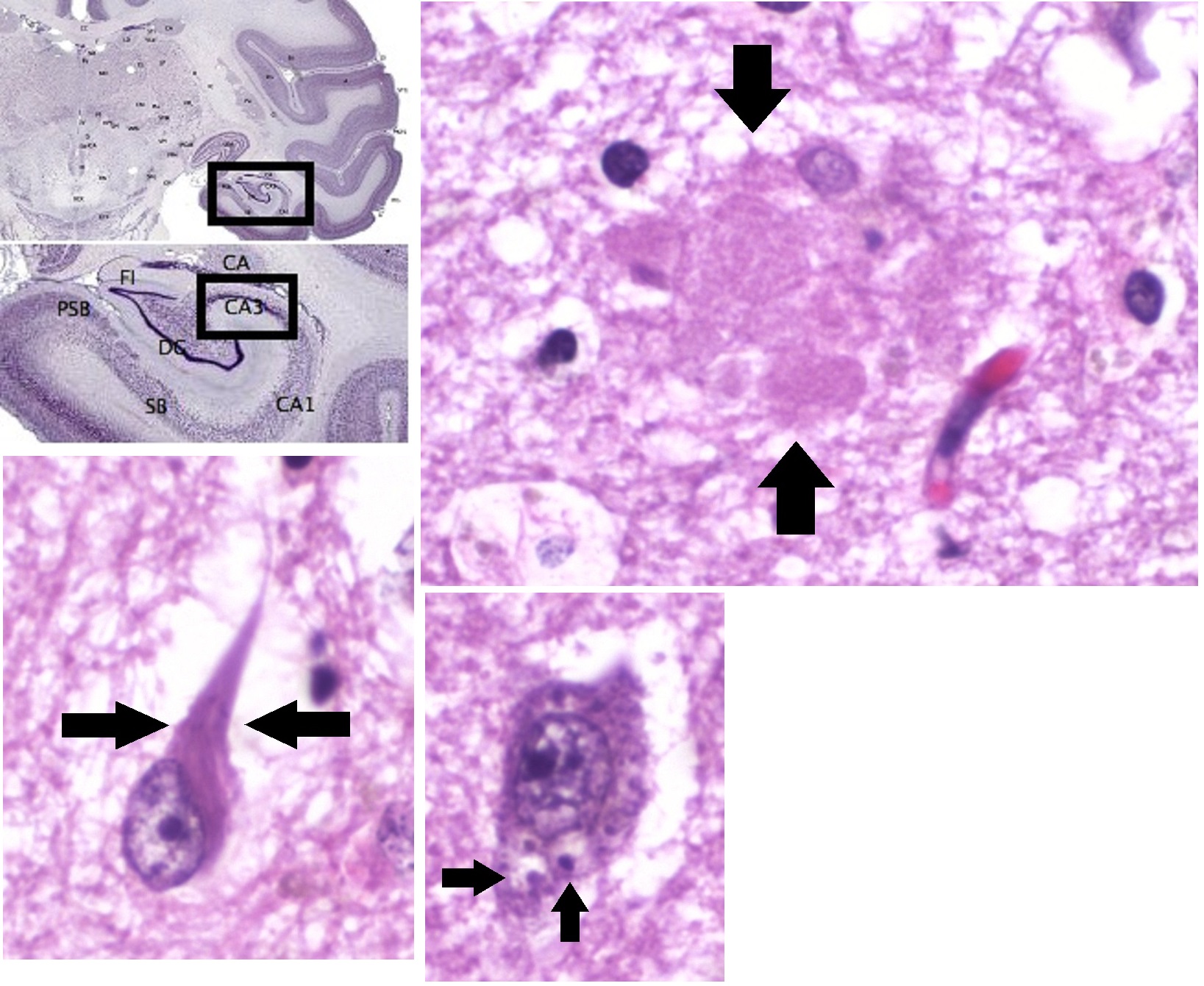
AD pathology is primarily characterized by the presence of senile plaques and neurofibrillary tangles. Plaques are made up of small peptides, typically 39–43 amino acids in length, called beta-amyloid (also written as A-beta or Aβ). Beta-amyloid is a fragment from a larger protein called amyloid precursor protein (APP), a transmembrane protein that penetrates through the neuron’s membrane. APP appears to play roles in normal neuron growth, survival and post-injury repair. APP is cleaved into smaller fragments by enzymes such as gamma secretase and beta secretase. One of these fragments gives rise to fibrils of beta-amyloid which can self-assemble into the dense extracellular deposits known as senile plaques or amyloid plaques.
14.1.2 Parkinson’s disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. It typically manifests as bradykinesia, rigidity, resting tremor and posture instability. The crude prevalence rate of PD has been reported to range from 15 per 100,000 to 12,500 per 100,000, and the incidence of PD from 15 per 100,000 to 328 per 100,000, with the disease being less common in Asian countries.
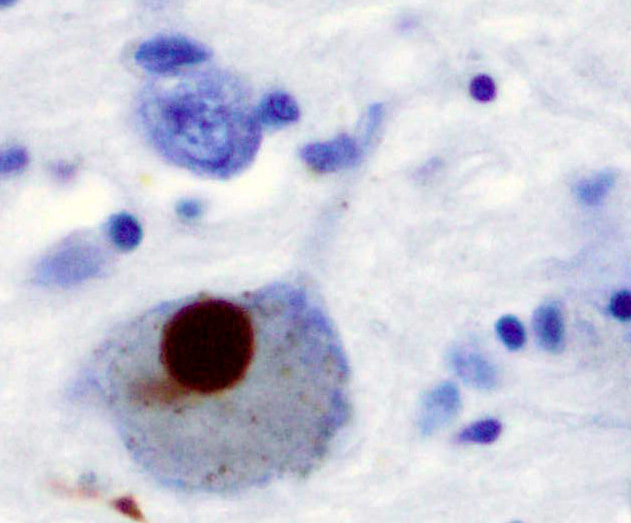
PD is primarily characterized by death of dopaminergic neurons in the substantia nigra, a region of the midbrain. The cause of this selective cell death is unknown. Notably, alpha-synuclein-ubiquitin complexes and aggregates are observed to accumulate in Lewy bodies within affected neurons. It is thought that defects in protein transport machinery and regulation, such as RAB1, may play a role in this disease mechanism. Impaired axonal transport of alpha-synuclein may also lead to its accumulation in Lewy bodies. Experiments have revealed reduced transport rates of both wild-type and two familial Parkinson’s disease-associated mutant alpha-synucleins through axons of cultured neurons. Membrane damage by alpha-synuclein could be another Parkinson’s disease mechanism.
The main known risk factor is age. Mutations in genes such as α-synuclein (SNCA), leucine-rich repeat kinase 2 (LRRK2), glucocerebrosidase (GBA), and tau protein (MAPT) can also cause hereditary PD or increase PD risk.
14.1.3 Huntington’s disease
Huntington’s disease (HD) is a rare autosomal dominant neurodegenerative disorder caused by mutations in the huntingtin gene. HD is characterized by loss of medium spiny neurons and astrogliosis. The first brain region to be substantially affected is the striatum, followed by degeneration of the frontal and temporal cortices. The striatum’s subthalamic nuclei send control signals to the globus pallidus, which initiates and modulates motion. The weaker signals from subthalamic nuclei thus cause reduced initiation and modulation of movement, resulting in the characteristic movements of the disorder, notably chorea.
HD is caused by polyglutamine tract expansion in the huntingtin gene, resulting in the aggregation-prone mutant huntingtin (mHtt). mHtt aggregates may be directly toxic. Additionally, they may damage molecular motors and microtubules to interfere with normal axonal transport, leading to impaired transport of important cargoes such as BDNF.
14.1.4 Amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) is a disease in which motor neurons are selectively targeted for degeneration. In 1993, missense mutations in the gene encoding the antioxidant enzyme Cu/Zn superoxide dismutase 1 (SOD1) were discovered in a subsets of patients with familial ALS. This discovery led researchers to focus on unlocking the mechanisms for SOD1-mediated diseases. However, the pathogenic mechanism underlying SOD1 mutant toxicity has yet to be resolved. More recently, TDP-43 and FUS protein aggregates have been implicated in some cases of the disease, and a mutation in chromosome 9 (C9orf72) is thought to be the most common known cause of sporadic ALS.
Recent independent research by Nagai et al. and Di Giorgio et al. provide in vitro evidence that the primary cellular sites where SOD1 mutations act are located on astrocytes. Astrocytes then cause the toxic effects on the motor neurons. The specific mechanism of toxicity still needs to be investigated, but the findings are significant because they implicate cells other than neuron cells in neurodegeneration.
14.2 Cerebrovascular Disease
Cerebrovascular disease includes a variety of medical conditions that affect the blood vessels of the brain and the cerebral circulation. Arteries supplying oxygen and nutrients to the brain are often damaged or deformed in these disorders. The most common presentation of cerebrovascular disease is an ischemic stroke or mini-stroke and sometimes a hemorrhagic stroke. Hypertension (high blood pressure) is the most important contributing risk factor for stroke and cerebrovascular diseases as it can change the structure of blood vessels and result in atherosclerosis. Atherosclerosis narrows blood vessels in the brain, resulting in decreased cerebral perfusion. Other risk factors that contribute to stroke include smoking and diabetes. Narrowed cerebral arteries can lead to ischemic stroke, but continually elevated blood pressure can also cause tearing of vessels, leading to a hemorrhagic stroke.
14.2.1 Stroke
A stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly. Signs and symptoms of a stroke may include an inability to move or feel on one side of the body, problems understanding or speaking, dizziness, or loss of vision to one side. Signs and symptoms often appear soon after the stroke has occurred. If symptoms last less than one or two hours, the stroke is a transient ischemic attack (TIA), also called a mini-stroke. A hemorrhagic stroke may also be associated with a severe headache. The symptoms of a stroke can be permanent. Long-term complications may include pneumonia and loss of bladder control.
Figure 14.4: CT-scan of the brain with an MCA infarct.
The main risk factor for stroke is high blood pressure. Other risk factors include tobacco smoking, obesity, high blood cholesterol, diabetes mellitus, a previous TIA, end-stage kidney disease, and atrial fibrillation. An ischemic stroke is typically caused by blockage of a blood vessel, though there are also less common causes. A hemorrhagic stroke is caused by either bleeding directly into the brain or into the space between the brain’s membranes. Bleeding may occur due to a ruptured brain aneurysm. Diagnosis is typically based on a physical exam and supported by medical imaging such as a CT scan or MRI scan. A CT scan can rule out bleeding, but may not necessarily rule out ischemia, which early on typically does not show up on a CT scan. Other tests such as an electrocardiogram (ECG) and blood tests are done to determine risk factors and rule out other possible causes. Low blood sugar may cause similar symptoms.
Prevention includes decreasing risk factors, surgery to open up the arteries to the brain in those with problematic carotid narrowing, and warfarin in people with atrial fibrillation. Aspirin or statins may be recommended by physicians for prevention. A stroke or TIA often requires emergency care. An ischemic stroke, if detected within three to four and half hours, may be treatable with a medication that can break down the clot. Some hemorrhagic strokes benefit from surgery. Treatment to attempt recovery of lost function is called stroke rehabilitation, and ideally takes place in a stroke unit; however, these are not available in much of the world.
In 2013, approximately 6.9 million people had an ischemic stroke and 3.4 million people had a hemorrhagic stroke. In 2015, there were about 42.4 million people who had previously had a stroke and were still alive. Between 1990 and 2010 the number of strokes which occurred each year decreased by approximately 10% in the developed world and increased by 10% in the developing world. In 2015, stroke was the second most frequent cause of death after coronary artery disease, accounting for 6.3 million deaths (11% of the total). About 3.0 million deaths resulted from ischemic stroke while 3.3 million deaths resulted from hemorrhagic stroke. About half of people who have had a stroke live less than one year. Overall, two thirds of strokes occurred in those over 65 years old.
Strokes can be classified into two major categories: ischemic and hemorrhagic. Ischemic strokes are caused by interruption of the blood supply to the brain, while hemorrhagic strokes result from the rupture of a blood vessel or an abnormal vascular structure. About 87% of strokes are ischemic, the rest being hemorrhagic. Bleeding can develop inside areas of ischemia, a condition known as “hemorrhagic transformation.” It is unknown how many hemorrhagic strokes actually start as ischemic strokes.
In the 1970s the World Health Organization defined stroke as a “neurological deficit of cerebrovascular cause that persists beyond 24 hours or is interrupted by death within 24 hours”, although the word “stroke” is centuries old. This definition was supposed to reflect the reversibility of tissue damage and was devised for the purpose, with the time frame of 24 hours being chosen arbitrarily. The 24-hour limit divides stroke from transient ischemic attack, which is a related syndrome of stroke symptoms that resolve completely within 24 hours. With the availability of treatments that can reduce stroke severity when given early, many now prefer alternative terminology, such as brain attack and acute ischemic cerebrovascular syndrome (modeled after heart attack and acute coronary syndrome, respectively), to reflect the urgency of stroke symptoms and the need to act swiftly.
In an ischemic stroke, blood supply to part of the brain is decreased, leading to dysfunction of the brain tissue in that area. There are four reasons why this might happen:
- Thrombosis (obstruction of a blood vessel by a blood clot forming locally)
- Embolism (obstruction due to an embolus from elsewhere in the body),
- Systemic hypoperfusion (general decrease in blood supply, e.g., in shock)
- Cerebral venous sinus thrombosis.
There are various classification systems for acute ischemic stroke. The Oxford Community Stroke Project classification (OCSP, also known as the Bamford or Oxford classification) relies primarily on the initial symptoms; based on the extent of the symptoms, the stroke episode is classified as total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), lacunar infarct (LACI) or posterior circulation infarct (POCI). These four entities predict the extent of the stroke, the area of the brain that is affected, the underlying cause, and the prognosis. The TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification is based on clinical symptoms as well as results of further investigations; on this basis, a stroke is classified as being due to (1) thrombosis or embolism due to atherosclerosis of a large artery, (2) an embolism originating in the heart, (3) complete blockage of a small blood vessel, (4) other determined cause, (5) undetermined cause (two possible causes, no cause identified, or incomplete investigation). Users of stimulants such as cocaine and methamphetamine are at a high risk for ischemic strokes.
There are two main types of hemorrhagic stroke:
- Intracerebral hemorrhage, which is basically bleeding within the brain itself (when an artery in the brain bursts, flooding the surrounding tissue with blood), due to either intraparenchymal hemorrhage (bleeding within the brain tissue) or intraventricular hemorrhage (bleeding within the brain’s ventricular system).
- Subarachnoid hemorrhage, which is basically bleeding that occurs outside of the brain tissue but still within the skull, and precisely between the arachnoid mater and pia mater (the delicate innermost layer of the three layers of the meninges that surround the brain).
The above two main types of hemorrhagic stroke are also two different forms of intracranial hemorrhage, which is the accumulation of blood anywhere within the cranial vault; but the other forms of intracranial hemorrhage, such as epidural hematoma (bleeding between the skull and the dura mater, which is the thick outermost layer of the meninges that surround the brain) and subdural hematoma (bleeding in the subdural space), are not considered “hemorrhagic strokes”.
Hemorrhagic strokes may occur on the background of alterations to the blood vessels in the brain, such as cerebral amyloid angiopathy, cerebral arteriovenous malformation and an intracranial aneurysm, which can cause intraparenchymal or subarachnoid hemorrhage.
In addition to neurological impairment, hemorrhagic strokes usually cause specific symptoms (for instance, subarachnoid hemorrhage classically causes a severe headache known as a thunderclap headache) or reveal evidence of a previous head injury.
Stroke symptoms typically start suddenly, over seconds to minutes, and in most cases do not progress further. The symptoms depend on the area of the brain affected. The more extensive the area of the brain affected, the more functions that are likely to be lost.
14.2.2 Aphasia
Aphasia is an inability to comprehend or formulate language because of damage to specific brain regions. Aphasia is most often caused by stroke, but any disease or damage to the parts of the brain that control language can cause aphasia. Some of these can include brain tumors, traumatic brain injury, and progressive neurological disorders.
People with aphasia may experience any of the following behaviors due to an acquired brain injury, although some of these symptoms may be due to related or concomitant problems, such as dysarthria or apraxia, and not primarily due to aphasia. Aphasia symptoms can vary based on the location of damage in the brain. Signs and symptoms may or may not be present in individuals with aphasia and may vary in severity and level of disruption to communication. Often those with aphasia will try to hide their inability to name objects by using words like thing. So when asked to name a pencil they may say it is a thing used to write.
Inability to comprehend language
Inability to pronounce, not due to muscle paralysis or weakness
Inability to speak spontaneously
Inability to form words
Inability to name objects (anomia)
Poor enunciation
Excessive creation and use of personal neologisms
Inability to repeat a phrase
Persistent repetition of one syllable, word, or phrase (stereotypies, recurrent/recurring utterances/speech automatism)
Paraphasia (substituting letters, syllables or words)
Agrammatism (inability to speak in a grammatically correct fashion)
Dysprosody (alterations in inflexion, stress, and rhythm)
Incomplete sentences
Inability to read
Inability to write
Limited verbal output
Difficulty in naming
Speech disorder
Speaking gibberish
Inability to follow or understand simple requests
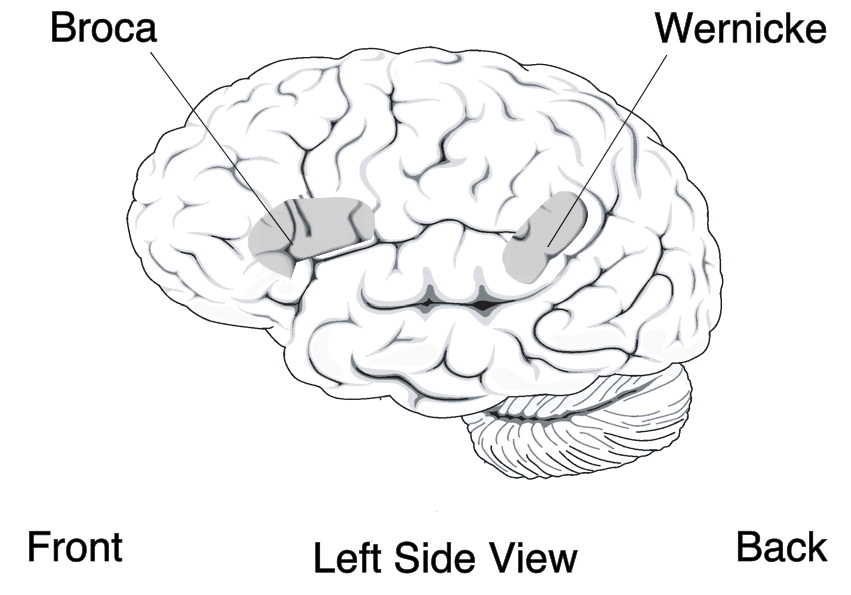
Localizationist approaches aim to classify the aphasias according to their major presenting characteristics and the regions of the brain that most probably gave rise to them. Inspired by the early work of nineteenth-century neurologists Paul Broca and Carl Wernicke, these approaches identify two major subtypes of aphasia and several more minor subtypes:
- Expressive aphasia (also known as “motor aphasia” or “Broca’s aphasia”), which is characterized by halted, fragmented, effortful speech, but well-preserved comprehension relative to expression. Damage is typically in the anterior portion of the left hemisphere, most notably Broca’s area. Individuals with Broca’s aphasia often have right-sided weakness or paralysis of the arm and leg, because the left frontal lobe is also important for body movement, particularly on the right side.
- Receptive aphasia (also known as “sensory aphasia” or “Wernicke’s aphasia”), which is characterized by fluent speech, but marked difficulties understanding words and sentences. Although fluent, the speech may lack in key substantive words (nouns, verbs, adjectives), and may contain incorrect words or even nonsense words. This subtype has been associated with damage to the posterior left temporal cortex, most notably Wernicke’s area. These individuals usually have no body weakness, because their brain injury is not near the parts of the brain that control movement.
- Conduction aphasia, where speech remains fluent, and comprehension is preserved, but the person may have disproportionate difficulty where repeating words or sentences. Damage typically involves the arcuate fasciculus and the left parietal region.
- Transcortical motor aphasia and transcortical sensory aphasia, which are similar to Broca’s and Wernicke’s aphasia respectively, but the ability to repeat words and sentences is disproportionately preserved.
Recent classification schemes adopting this approach, such as the Boston-Neoclassical Model, also group these classical aphasia subtypes into two larger classes: the nonfluent aphasias (which encompasses Broca’s aphasia and transcortical motor aphasia) and the fluent aphasias (which encompasses Wernicke’s aphasia, conduction aphasia and transcortical sensory aphasia). These schemes also identify several further aphasia subtypes, including: anomic aphasia, which is characterized by a selective difficulty finding the names for things; and global aphasia, where both expression and comprehension of speech are severely compromised.
Many localizationist approaches also recognize the existence of additional, more “pure” forms of language disorder that may affect only a single language skill. For example, in pure alexia, a person may be able to write but not read, and in pure word deafness, they may be able to produce speech and to read, but not understand speech when it is spoken to them.
14.2.3 Prosopagnosia
Prosopagnosia (from Greek prósōpon, meaning “face”, and agnōsía, meaning “non-knowledge”), also called face blindness, is a cognitive disorder of face perception in which the ability to recognize familiar faces, including one’s own face (self-recognition), is impaired, while other aspects of visual processing (e.g., object discrimination) and intellectual functioning (e.g., decision-making) remain intact. The term originally referred to a condition following acute brain damage (acquired prosopagnosia), but a congenital or developmental form of the disorder also exists, with a prevalence rate of 2.5%. The specific brain area usually associated with prosopagnosia is the fusiform gyrus, which activates specifically in response to faces. The functionality of the fusiform gyrus allows most people to recognize faces in more detail than they do similarly complex inanimate objects. For those with prosopagnosia, the new method for recognizing faces depends on the less sensitive object-recognition system. The right hemisphere fusiform gyrus is more often involved in familiar face recognition than the left. It remains unclear whether the fusiform gyrus is only specific for the recognition of human faces or if it is also involved in highly trained visual stimuli.
Acquired prosopagnosia results from occipito-temporal lobe damage and is most often found in adults. This is further subdivided into apperceptive and associative prosopagnosia. In congenital prosopagnosia, the individual never adequately develops the ability to recognize faces.
Though there have been several attempts at remediation, no therapies have demonstrated lasting real-world improvements across a group of prosopagnosics. Prosopagnosics often learn to use “piecemeal” or “feature-by-feature” recognition strategies. This may involve secondary clues such as clothing, gait, hair color, skin color, body shape, and voice. Because the face seems to function as an important identifying feature in memory, it can also be difficult for people with this condition to keep track of information about people, and socialize normally with others. Prosopagnosia has also been associated with other disorders that are associated with nearby brain areas: left hemianopsia (loss of vision from left side of space, associated with damage to the right occipital lobe), achromatopsia (a deficit in color perception often associated with unilateral or bilateral lesions in the temporo-occipital junction) and topographical disorientation (a loss of environmental familiarity and difficulties in using landmarks, associated with lesions in the posterior part of the parahippocampal gyrus and anterior part of the lingual gyrus of the right hemisphere).
14.2.4 Hemispatial neglect
Hemispatial neglect is a neuropsychological condition in which, after damage to one hemisphere of the brain is sustained, a deficit in attention to and awareness of one side of the field of vision is observed. It is defined by the inability of a person to process and perceive stimuli on one side of the body or environment, where that inability is not due to a lack of sensation. Hemispatial neglect is very commonly contralateral to the damaged hemisphere, but instances of ipsilesional neglect (on the same side as the lesion) have been reported.
Hemispatial neglect results most commonly from strokes and brain unilateral injury to the right cerebral hemisphere, with rates in the critical stage of up to 80% causing visual neglect of the left-hand side of space. Neglect is often produced by massive strokes in the middle cerebral artery region and is variegated, so that most sufferers do not exhibit all of the syndrome’s traits. Right-sided spatial neglect is rare because there is redundant processing of the right space by both the left and right cerebral hemispheres, whereas in most left-dominant brains the left space is only processed by the right cerebral hemisphere. Although it most strikingly affects visual perception (‘visual neglect’), neglect in other forms of perception can also be found, either alone or in combination with visual neglect.
For example, a stroke affecting the right parietal lobe of the brain can lead to neglect for the left side of the visual field, causing a patient with neglect to behave as if the left side of sensory space is nonexistent (although they can still turn left). In an extreme case, a patient with neglect might fail to eat the food on the left half of their plate, even though they complain of being hungry. If someone with neglect is asked to draw a clock, their drawing might show only the numbers 12 to 6, or all 12 numbers might be on one half of the clock face with the other half distorted or blank. Neglect patients may also ignore the contralesional side of their body; for instance, they might only shave, or apply make-up to, the non-neglected side. These patients may frequently collide with objects or structures such as door frames on the side being neglected.
Neglect may also present as a delusional form, where the patient denies ownership of a limb or an entire side of the body. Since this delusion often occurs alone, without the accompaniment of other delusions, it is often labeled as a monothematic delusion.
Neglect not only affects present sensation but memory and recall perception as well. A patient suffering from neglect may also, when asked to recall a memory of a certain object and then draw said object, draw only half of the object. It is unclear, however, if this is due to a perceptive deficit of the memory (to the patient having lost pieces of spatial information of the memory) or whether the information within the memory is whole and intact but simply being ignored, the same way portions of a physical object in the patient’s presence would be ignored.
Some forms of neglect may also be very mild—for example, in a condition called extinction where competition from the ipsilesional stimulus impedes perception of the contralesional stimulus. These patients, when asked to fixate on the examiner’s nose, can detect fingers being wiggled on the affected side. If the examiner were to wiggle his or her fingers on both the affected and unaffected sides of the patient, the patient will report seeing movement only on the ipsilesional side.
14.3 Epilepsy
Epilepsy is a group of neurological disorders characterized by recurrent epileptic seizures. Epileptic seizures are episodes that can vary from brief and nearly undetectable periods to long periods of vigorous shaking. These episodes can result in physical injuries, including occasionally broken bones. In epilepsy, seizures have a tendency to recur and, as a rule, have no immediate underlying cause. Isolated seizures that are provoked by a specific cause such as poisoning are not deemed to represent epilepsy. People with epilepsy may be treated differently in various areas of the world and experience varying degrees of social stigma due to their condition.
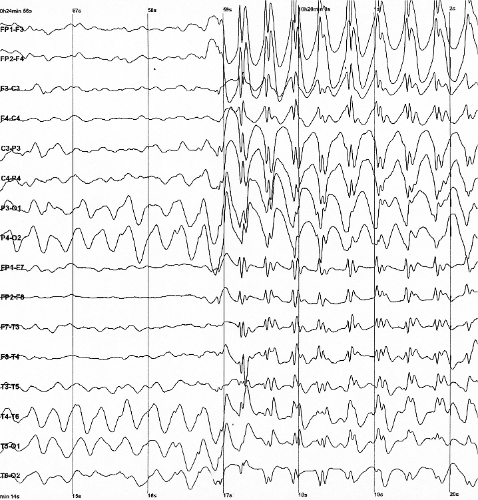
Figure 14.6: The electroencephalogram recording of a person with childhood absence epilepsy showing a seizure. The waves are black on a white background.
The underlying mechanism of epileptic seizures is excessive and abnormal neuronal activity in the cortex of the brain. The reason this occurs in most cases of epilepsy is unknown. Some cases occur as the result of brain injury, stroke, brain tumors, infections of the brain, or birth defects through a process known as epileptogenesis. Known genetic mutations are directly linked to a small proportion of cases. The diagnosis involves ruling out other conditions that might cause similar symptoms, such as fainting, and determining if another cause of seizures is present, such as alcohol withdrawal or electrolyte problems. This may be partly done by imaging the brain and performing blood tests. Epilepsy can often be confirmed with an electroencephalogram (EEG), but a normal test does not rule out the condition.
Epilepsy that occurs as a result of other issues may be preventable. Seizures are controllable with medication in about 70% of cases; inexpensive anti-seizure medications are often available. In those whose seizures do not respond to medication, surgery, neurostimulation or dietary changes may then be considered. Not all cases of epilepsy are lifelong, and many people improve to the point that treatment is no longer needed.
As of 2015, about 39 million people have epilepsy. Nearly 80% of cases occur in the developing world. In 2015, it resulted in 125,000 deaths, an increase from 112,000 in 1990. Epilepsy is more common in older people. In the developed world, onset of new cases occurs most frequently in babies and the elderly. In the developing world, onset is more common in older children and young adults due to differences in the frequency of the underlying causes. About 5–10% of people will have an unprovoked seizure by the age of 80, and the chance of experiencing a second seizure is between 40% and 50%. In many areas of the world, those with epilepsy either have restrictions placed on their ability to drive or are not permitted to drive until they are free of seizures for a specific length of time. The word epilepsy is from Ancient Greek ἐπιλαμβάνειν, ‘to seize, possess, or afflict’.
Normally brain electrical activity is non-synchronous, as neurons do not normally fire in sync with each other, but rather fire in order as signals travel throughout the brain. Its activity is regulated by various factors both within the neuron and the cellular environment. Factors within the neuron include the type, number and distribution of ion channels, changes to receptors and changes of gene expression. Factors around the neuron include ion concentrations, synaptic plasticity and regulation of transmitter breakdown by glial cells. Chronic inflammation also appears to play a role.
The exact mechanism of epilepsy is unknown, but a little is known about its cellular and network mechanisms. However, it is unknown under which circumstances the brain shifts into the activity of a seizure with its excessive synchronization.
In epilepsy, the resistance of excitatory neurons to fire during this period is decreased. This may occur due to changes in ion channels or inhibitory neurons not functioning properly. This then results in a specific area from which seizures may develop, known as a “seizure focus”. Another mechanism of epilepsy may be the up-regulation of excitatory circuits or down-regulation of inhibitory circuits following an injury to the brain. These secondary epilepsies occur through processes known as epileptogenesis. Failure of the blood–brain barrier may also be a causal mechanism as it would allow substances in the blood to enter the brain.
There is evidence that epileptic seizures are usually not a random event. Seizures are often brought on by factors such as stress, alcohol abuse, flickering light, or a lack of sleep, among others. The term seizure threshold is used to indicate the amount of stimulus necessary to bring about a seizure. Seizure threshold is lowered in epilepsy.
In epileptic seizures a group of neurons begin firing in an abnormal, excessive, and synchronized manner. This results in a wave of depolarization known as a paroxysmal depolarizing shift. Normally, after an excitatory neuron fires it becomes more resistant to firing for a period of time. This is due in part to the effect of inhibitory neurons, electrical changes within the excitatory neuron, and the negative effects of adenosine.
Focal seizures begin in one hemisphere of the brain while generalized seizures begin in both hemispheres. Some types of seizures may change brain structure, while others appear to have little effect. Gliosis, neuronal loss, and atrophy of specific areas of the brain are linked to epilepsy but it is unclear if epilepsy causes these changes or if these changes result in epilepsy.
The diagnosis of epilepsy is typically made based on observation of the seizure onset and the underlying cause. An electroencephalogram (EEG) to look for abnormal patterns of brain waves and neuroimaging (CT scan or MRI) to look at the structure of the brain are also usually part of the workup. While figuring out a specific epileptic syndrome is often attempted, it is not always possible. Video and EEG monitoring may be useful in difficult cases.
14.4 Mental Disorders
A mental disorder, also called a mental illness or psychiatric disorder, is a behavioral or mental pattern that causes significant distress or impairment of personal functioning. Such features may be persistent, relapsing and remitting, or occur as a single episode. Many disorders have been described, with signs and symptoms that vary widely between specific disorders. Such disorders may be diagnosed by a mental health professional.
The causes of mental disorders are often unclear. Theories may incorporate findings from a range of fields. Mental disorders are usually defined by a combination of how a person behaves, feels, perceives, or thinks. This may be associated with particular regions or functions of the brain, often in a social context. A mental disorder is one aspect of mental health. Cultural and religious beliefs, as well as social norms, should be taken into account when making a diagnosis.
Services are based in psychiatric hospitals or in the community, and assessments are carried out by mental health professionals such as psychiatrists, psychologists, psychiatric nurses and clinical social workers, using various methods such as psychometric tests but often relying on observation and questioning. Treatments are provided by various mental health professionals. Psychotherapy and psychiatric medication are two major treatment options. Other treatments include lifestyle changes, social interventions, peer support, and self-help. In a minority of cases, there might be involuntary detention or treatment. Prevention programs have been shown to reduce depression.
Common mental disorders include depression, which affects about 264 million, bipolar disorder, which affects about 45 million, dementia, which affects about 50 million, and schizophrenia and other psychoses, which affects about 20 million people globally. Developmental disorders include intellectual disability and pervasive developmental disorders which usually arise in infancy or childhood. Stigma and discrimination can add to the suffering and disability associated with mental disorders, leading to various social movements attempting to increase understanding and challenge social exclusion.
14.4.1 Mood Disorders
Mood disorder, also known as mood affective disorders, is a group of conditions where a disturbance in the person’s mood is the main underlying feature. The classification is in the Diagnostic and Statistical Manual of Mental Disorders (DSM) and International Classification of Diseases (ICD).
Mood disorders fall into the basic groups of elevated mood, such as mania or hypomania; depressed mood, of which the best-known and most researched is major depressive disorder (MDD) (commonly called clinical depression, unipolar depression, or major depression); and moods which cycle between mania and depression, known as bipolar disorder (BD) (formerly known as manic depression). There are several sub-types of depressive disorders or psychiatric syndromes featuring less severe symptoms such as dysthymic disorder (similar to but milder than MDD) and cyclothymic disorder (similar to but milder than BD). Mood disorders may also be substance induced or occur in response to a medical condition.
English psychiatrist Henry Maudsley proposed an overarching category of affective disorder. The term was then replaced by mood-disorder, as the latter term refers to the underlying or longitudinal emotional state, whereas the former refers to the external expression observed by others.
Major depressive disorder (MDD), commonly called major depression, unipolar depression, or clinical depression, wherein a person has one or more major depressive episodes. After a single episode, Major Depressive Disorder (single episode) would be diagnosed. After more than one episode, the diagnosis becomes Major Depressive Disorder (Recurrent). Depression without periods of mania is sometimes referred to as unipolar depression because the mood remains at the bottom “pole” and does not climb to the higher, manic “pole” as in bipolar disorder.
Individuals with a major depressive episode or major depressive disorder are at increased risk for suicide. Seeking help and treatment from a health professional dramatically reduces the individual’s risk for suicide. Studies have demonstrated that asking if a depressed friend or family member has thought of committing suicide is an effective way of identifying those at risk, and it does not “plant” the idea or increase an individual’s risk for suicide in any way. Epidemiological studies carried out in Europe suggest that, at this moment, roughly 8.5 percent of the world’s population have a depressive disorder. No age group seems to be exempt from depression, and studies have found that depression appears in infants as young as 6 months old who have been separated from their mothers.
Bipolar disorder (BD) (also called “manic depression” or “manic-depressive disorder”), an unstable emotional condition characterized by cycles of abnormal, persistent high mood (mania) and low mood (depression), which was formerly known as “manic depression” (and in some cases rapid cycling, mixed states, and psychotic symptoms). It is estimated that roughly 1% of the adult population has bipolar I, a further 1% has bipolar II or cyclothymia, and somewhere between 2% and 5% percent have “sub-threshold” forms of bipolar disorder. Furthermore, the possibility of getting bipolar disorder when one parent is diagnosed with it is 15–30%. Risk, when both parents have it, is 50–75%. Also, while with bipolar siblings the risk is 15–25%, with identical twins it is about 70%. A minority of people with bipolar disorder have high creativity, artistry or a particular gifted talent. Before the mania phase becomes too extreme, its energy, ambition, enthusiasm and grandiosity often bring people with this type of mood disorder life’s masterpieces.
14.4.2 Schizophrenia
Schizophrenia is a psychiatric disorder characterized by continuous or relapsing episodes of psychosis. Major symptoms include hallucinations (typically hearing voices), delusions, and disorganized thinking. Other symptoms include social withdrawal, decreased emotional expression, and apathy. Symptoms typically come on gradually, begin in young adulthood, and in many cases never resolve. There is no objective diagnostic test; diagnosis is based on observed behavior, a history that includes the person’s reported experiences, and reports of others familiar with the person. To be diagnosed with schizophrenia, symptoms and functional impairment need to be present for six months (DSM-5) or one month (ICD-11). Many people with schizophrenia have other mental disorders that often includes an anxiety disorder such as panic disorder, an obsessive–compulsive disorder, or a substance use disorder.
About 0.3% to 0.7% of people are affected by schizophrenia during their lifetime. In 2017, there were an estimated 1.1 million new cases and in 2019 a total of 20 million cases globally. Males are more often affected and on average have an earlier onset. The causes of schizophrenia include genetic and environmental factors. Genetic factors include a variety of common and rare genetic variants. Possible environmental factors include being raised in a city, cannabis use during adolescence, infections, the ages of a person’s mother or father, and poor nutrition during pregnancy.
About half of those diagnosed with schizophrenia will have a significant improvement over the long term with no further relapses, and a small proportion of these will recover completely. The other half will have a lifelong impairment, and severe cases may be repeatedly admitted to hospital. Social problems such as long-term unemployment, poverty, homelessness, exploitation, and victimization are common consequences of schizophrenia. Compared to the general population, people with schizophrenia have a higher suicide rate (about 5% overall) and more physical health problems, leading to an average decreased life expectancy of 20 years. In 2015, an estimated 17,000 deaths were caused by schizophrenia.
The mainstay of treatment is antipsychotic medication, along with counselling, job training, and social rehabilitation. Up to a third of people do not respond to initial antipsychotics, in which case the antipsychotic clozapine may be used. In situations where there is a risk of harm to self or others, a short involuntary hospitalization may be necessary. Long-term hospitalization may be needed for a small number of people with severe schizophrenia. In countries where supportive services are limited or unavailable, long-term hospital stays are more typical.
Schizophrenia is a mental disorder characterized by significant alterations in perception, thoughts, mood, and behavior. Symptoms are described in terms of positive, negative, and cognitive symptoms. The positive symptoms of schizophrenia are the same for any psychosis and are sometimes referred to as psychotic symptoms. These may be present in any of the different psychoses, and are often transient making early diagnosis of schizophrenia problematic. Psychosis noted for the first time in a person who is later diagnosed with schizophrenia is referred to as a first-episode psychosis (FEP).
Positive symptoms are those symptoms that are not normally experienced, but are present in people during a psychotic episode in schizophrenia. They include delusions, hallucinations, and disorganized thoughts and speech, typically regarded as manifestations of psychosis. Hallucinations most commonly involve the sense of hearing as hearing voices but can sometimes involve any of the other senses of taste, sight, smell, and touch. They are also typically related to the content of the delusional theme. Delusions are bizarre or persecutory in nature. Distortions of self-experience such as feeling as if one’s thoughts or feelings are not really one’s own, to believing that thoughts are being inserted into one’s mind, sometimes termed passivity phenomena, are also common. Thought disorders can include thought blocking, and disorganized speech – speech that is not understandable is known as word salad. Positive symptoms generally respond well to medication, and become reduced over the course of the illness, perhaps related to the age-related decline in dopamine activity.
Negative symptoms are deficits of normal emotional responses, or of other thought processes. The five recognised domains of negative symptoms are: blunted affect – showing flat expressions or little emotion; alogia – a poverty of speech; anhedonia – an inability to feel pleasure; asociality – the lack of desire to form relationships, and avolition – a lack of motivation and apathy. Avolition and anhedonia are seen as motivational deficits resulting from impaired reward processing. Reward is the main driver of motivation and this is mostly mediated by dopamine. It has been suggested that negative symptoms are multidimensional and they have been categorised into two subdomains of apathy or lack of motivation, and diminished expression. Apathy includes avolition, anhedonia, and social withdrawal; diminished expression includes blunt effect, and alogia. Sometimes diminished expression is treated as both verbal and non-verbal. Apathy accounts for around 50 per cent of the most often found negative symptoms and affects functional outcome and subsequent quality of life. Apathy is related to disrupted cognitive processing affecting memory and planning including goal-directed behaviour. The two subdomains has suggested a need for separate treatment approaches. A lack of distress – relating to a reduced experience of depression and anxiety is another noted negative symptom. A distinction is often made between those negative symptoms that are inherent to schizophrenia, termed primary; and those that result from positive symptoms, from the side effects of antipsychotics, substance abuse, and social deprivation - termed secondary negative symptoms. Negative symptoms are less responsive to medication and the most difficult to treat. However if properly assessed, secondary negative symptoms are amenable to treatment.
Scales for specifically assessing the presence of negative symptoms, and for measuring their severity, and their changes have been introduced since the earlier scales such as the PANNS that deals with all types of symptoms. These scales are the Clinical Assessment Interview for Negative Symptoms (CAINS), and the Brief Negative Symptom Scale (BNSS) also known as second-generation scales. In 2020, ten years after its introduction a cross-cultural study of the use of BNSS found valid and reliable psychometric evidence for the five-domain structure cross-culturally. The BNSS is designed to assess both the presence and severity and change of negative symptoms of the five recognised domains, and the additional item of reduced normal distress. BNSS can register changes in negative symptoms in relation to psychosocial and pharmacological intervention trials. BNSS has also been used to study a proposed non-D2 treatment called SEP-363856. Findings supported the favouring of five domains over the two-dimensional proposition.
Cognitive deficits are the earliest and most constantly found symptoms in schizophrenia. They are often evident long before the onset of illness in the prodromal stage, and may be present in early adolescence, or childhood. They are a core feature but not considered to be core symptoms, as are positive and negative symptoms. However, their presence and degree of dysfunction is taken as a better indicator of functionality than the presentation of core symptoms. Cognitive deficits become worse at first episode psychosis but then return to baseline, and remain fairly stable over the course of the illness.
The deficits in cognition are seen to drive the negative psychosocial outcome in schizophrenia, and are claimed to equate to a possible reduction in IQ from the norm of 100 to 70–85. Cognitive deficits may be of neurocognition (nonsocial) or of social cognition. Neurocognition is the ability to receive and remember information, and includes verbal fluency, memory, reasoning, problem solving, speed of processing, and auditory and visual perception. Verbal memory and attention are seen to be the most affected. Verbal memory impairment is associated with a decreased level of semantic processing (relating meaning to words). Another memory impairment is that of episodic memory. An impairment in visual perception that is consistently found in schizophrenia is that of visual backward masking. Visual processing impairments include an inability to perceive complex visual illusions. Social cognition is concerned with the mental operations needed to interpret, and understand the self and others in the social world. This is also an associated impairment, and facial emotion perception is often found to be difficult. Facial perception is critical for ordinary social interaction. Cognitive impairments do not usually respond to antipsychotics, and there are a number of interventions that are used to try to improve them; cognitive remediation therapy has been found to be of particular help.
Onset typically occurs between the late teens and early 30s, with the peak incidence occurring in males in the early to mid twenties, and in females in the late twenties. Onset before the age of 17 is known as early-onset, and before the age of 13, as can sometimes occur is known as childhood schizophrenia or very early-onset. A later stage of onset can occur between the ages of 40 and 60, known as late-onset schizophrenia. A later onset over the age of 60 which may be difficult to differentiate as schizophrenia, is known as very-late-onset schizophrenia-like psychosis. Late onset has shown that a higher rate of females are affected; they have less severe symptoms, and need lower doses of antipsychotics. The earlier favouring of onset in males is later seen to be balanced by a post-menopausal increase in the development in females. Estrogen produced pre-menopause, has a dampening effect on dopamine receptors but its protection can be overridden by a genetic overload. There has been a dramatic increase in the numbers of older adults with schizophrenia. An estimated 70% of those with schizophrenia have cognitive deficits, and these are most pronounced in early onset, and late-onset illness.
Onset may happen suddenly, or may occur after the slow and gradual development of a number of signs and symptoms in a period known as the prodromal stage. Up to 75% of those with schizophrenia go through a prodromal stage. The negative and cognitive symptoms in the prodrome can precede FEP by many months, and up to five years. The period from FEP and treatment is known as the duration of untreated psychosis (DUP) which is seen to be a factor in functional outcome. The prodromal stage is the high-risk stage for the development of psychosis. Since the progression to first episode psychosis, is not inevitable an alternative term is often preferred of at risk mental state" Cognitive dysfunction at an early age impacts on a young person’s ususal cognitive development. Recognition and early intervention at the prodromal stage would minimize the associated disruption to educational and social development, and has been the focus of many studies. It is suggested that the use of anti-inflammatory compounds such as D-serine may prevent the transition to schizophrenia. Cognitive symptoms are not secondary to positive symptoms, or to the side effects of antipsychotics.
Cognitive impairments in the prodromal stage become worse after first episode psychosis (after which they return to baseline and then remain fairly stable), making early intervention to prevent such transition of prime importance. Early treatment with cognitive behavioral therapies are the gold standard. Neurological soft signs of clumsiness and loss of fine motor movement are often found in schizophrenia, and these resolve with effective treatment of FEP.
Genetic, environmental, and vulnerability factors are involved in the development of schizophrenia. The interactions of these risk factors are complex, as numerous and diverse insults from conception to adulthood can be involved. A genetic predisposition on its own, without interacting environmental factors, will not give rise to the development of schizophrenia. Schizophrenia is described as a neurodevelopmental disorder that lacks a precise boundary in its definition.